The world of herbalism has an infinite barrage of information to spray at you, which might seem overwhelming. The way to make this info managable and usable is to find the patterns and interrelations within it. That way you don’t have to memorise an impossible amount of data. It’s a good way to attack all of the large bodies of knowledge we must take in. Today we’ll look at the relatedness of some herbs by focusing on a molecule that they share, and how you can use more of your senses to find related plants, cement them into your brain, and more quickly gain abilities to choose the most appropriate alternatives when you need them.
One of the last plants to give up its leaves in a weedom Winter is thyme, and it usually recovers in Spring. Lately in our region it’s almost evergreen. This herb compliments many foods, and is added lots of meat and vegetable dishes in our kitchen. It’s a primary source of the long revered and utilized thymol, though numerous other related plants also produce this compound in quantity. We won’t be concentrating on the single plant, but rather a group of them which are tied together by their production of thymol and related compounds.
There are many different types of herbalists, some of whom seem not at all concerned with the biochemistry of their formulary of best weeds, but who still know, by their characteristics, which ones are related to each other and to your needs. They’ve experienced the medicinal properties of the plants through their physical properties and their taste. They won’t tell you all about polysaccharides, but instead might tell you about mucilage, demulcents, gels, and sliminess, and maybe coolness and sweetness. We can refer back to Rosalee De la Foret as an example of one who systematized a way for you to learn about a lot of herbs through their tastes, and tying this to their functions. But you know what brings the tastes: Molecules.
Our pharmacognosy friends can classify the molecules by chemical category and can tell you from that standpoint why a particular plant is
salty, usually from inorganic compounds and minerals
sweet, from sugars and polysaccharides
bitter, from alkaloids and certain phenolics and steroids.
sour, usually from organic acids, such as the famous citric acid
umami, (savory) due to certain amino acids and nucleotides,
and astringent, a very special mouth feel which is not quite a taste, but a puckery or “hairy tongue” feeling of dryness that generally comes from tannins. Rosalee used these patterns to help teach people to recognize some of the herbal functions by taste. Likewise the relatedness of various plants by their chemistry can be determined by taste testing.
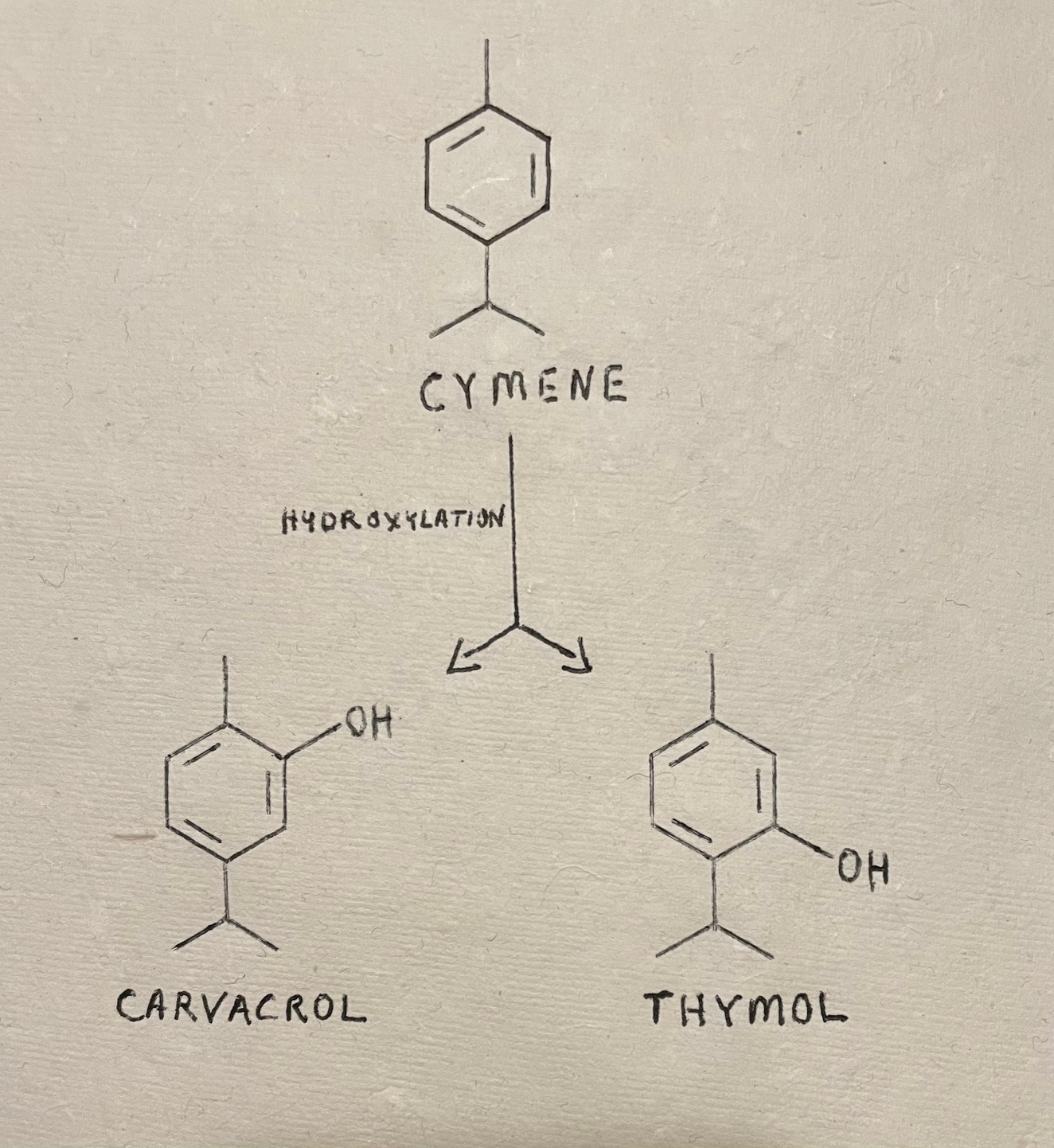
Thymol ties numerous useful herbs together in the same flavor family, even if they might not be in the same plant family. This compound is widely distributed among the mint family, Lamiaceae. Monarda species - bee balm, and Thymus vulgaris contain some of the highest proportions of thymol in their essential oils. Numerous other mint species also offer up varying amounts: oregano (Origanum vulgare), marjoram (Organum majorana), the many types of basil (Ocimium species), and savory (Satureja species). Thymol is also offered up by plants within the families Apiaceae by an Indian or Middle eastern spice called ajwain (Trachyspermium ammi or Carum copticum), yerba mansa (Anemopsis californica), a famous, Southwestern antimicrobial member of the family Saururaceae), and Lippia species of Verbenaceae. Thymol has also been found among members of families Scrophulariaceae and Ranunculaceae. Thymol, frequently accompanied by its close molecular buddy, carvacrol, is widely found protecting various plants from infectious diseases and insect assaults. Both of these compounds are produced metabolically by hydroxylation of cymene, and all 3 of them contribute to the antimicrobial, anti-inflammatory, and numerous other salutary effects of innumerable herbs.
A taste or smell-a-tour among the mint family will reveal the thymol containing plants, and train you to detect this compound in a wider variety of sources. You’ll also smell and taste it in an astonishing array of commercial products, including those used for cleaning and sanitation, dental products and mouthwashes, (yaa, thymol is one of the essential oil components used in Listerine), topical acne medications, food preservatives, and numerous agricultural uses.
Commercially Thymus vulgaris is one of the most productive sources for producing thymol. This plant is native to the Middle East area, and was originally used thousands of years ago for mostly medicinal purposes. Egyptians took advantage of the antimicrobial properties and used it for embalming mummies, but also to flavor food. In Rome it was considered an aphrodisiac. In ancient times it was also seen as a sort of antidote, and was eaten before meals to prevent sickness. Perhaps this was a means to avoid some food poisonings. During the Middle Ages, thyme was used to address digestive, respiratory and skin ailments. Later it was applied topically for the plague. In the 1700s, thymol is said to have been first extracted from the plant. Italians discovered that it had some activity against hookworms. Thyme traveled to the U.S. in the 1800s as medicine but did not gain big popularity as a culinary herb until the last 100 or so years.
Big interest in thymol persists due to its own medicinal activities but also for its use to manufacture myriad other compounds of medicinal and industrial use. It has most significant importance in the semi-synthetic production of menthol, the vapor of which pervades our lives. Hydrodistillation has been a traditional method of obtaining the crystalline thymol, but super critical fluid extraction using CO2 is of highest current interest, because it is done at cold temperatures and is is therefore less destructive and a cleaner process. Good yields have been obtained, by this method, in India from the herb, ajwain.
Much of the antimicrobial activity of thymol, which affects both gram negative and gram positive bacteria, have to do with the disruption of their abilities to form biofilms. This impedes bacterial communication and ability to spread, and is promising for use to increase the effects of existing antibiotics against resistant microorganisms. The integrity of bacterial cell membranes is also affected.
Antifungal activity of thymol against numerous species is mediated by an effect on fungal cell membrane formation and integrity perhaps by affecting fatty acid metabolism and ergosterol production. Negative effects on biofilm production may also contribute to the activity, at much less toxic doses than those of the classic anti-fungal, amphotericin (nicknamed ampho-terrible in medicine). Research has been done showing some hope in being able to better address cryptococcal infections in immunosuppressed patients.
Long industrial interest in thymol as a disinfectant and preservative, as well as the medical interests have led to extensive testing for genotoxicity. This leaves us with numerous, easy to grow sources of considerable benefit, which are known to be comparatively safe.
The main barrier to the use of thymol and its related compounds for systemic medicinal use is the low water solubility and bioavailability. Numerous methods of glycosylation or encapsulation in lipid or carbohydrate micelles, have been employed to overcome this, which have shown promising results. Wherever thymol can be delivered directly, the GI tract or by inhalation, or topically, the traditional, medicinal uses have proliferated. Thymol has been noted to increase the absorption of numerous other drugs through the skin, and to lower the melting point of lidocaine in mixture. Forming eutectic mixtures of various drugs with menthol or thymol, etc. allows them to be distributed into an ointment or other vehicle, and can allow them to become topically absorbed into skin. Physical properties, which cause a mixture of 2 solid substances to become liquid at room temperature, can allow efficient compounding with otherwise insoluble medicinal materials. Some of this can be done in the home with no special equipment.
Thymol containing cleaning solutions were added to the lists of acceptable surface disinfectants in the US and Canada amid all the product shortages during the COVID lockdowns, and accorded a very high safety rating. (Many of the other hospital disinfectants in common use are quite irritating to the skin, nasal mucosa and lungs.)
The inhaled anaesthetic drug, Fluothane (halothane) contains 0.01% (by weight) of thymol as a stabilizing agent. The action of thymol in this case is as an antioxidant to prevent the degradation of the halothane product into caustic substances, rather than as an antimicrobial. Since the thymol crystallizes at room temp, it can gum up the aerosolizers, and there’s quite a bit of available literature concerning the need to clean them out from time to time to maintain a proper halothane flow rate for anaesthesia.
Last but not least, thymol and its molecular companions in their natural state exhibit delectable flavor for culinary use, pleasing odors for the perfumery industry, and smooth muscle relaxant effects which can ease digestion. They remain well used in Europe for addressing spasmodic cough and aiding expectoration of sticky mucus.
By no means did we give an exhaustive list of the plants which produce thymol, nor a complete listing of its known benefits. It can be a continuing adventure for you to identify more of the common foods and spices which contribute this compound to your life.
Where we Dig
1. Boskabady MH, Alitaneh S, Alavinezhad A. Carum copticum L.: A Herbal Medicine with Various Pharmacological Effects. Biomed Res Int. 2014;2014:569087. doi:10.1155/2014/569087
2. Going Deeper with Yerba Mansa | Albuquerque Herbalism. Accessed January 23, 2024. https://albuquerqueherbalism.com/2016/10/11/going-deeper-with-yerba-mansa/
3. Ganora L. Herbal Constituents, 2nd Edition - Foundations of Phytochemistry. Lulu Press, Inc.; 2021. https://openlibrary.org/books/OL35083771M/Herbal_Constituents_2nd_Edition
4. Lee S, UMANO K, Shibamoto T, Lee S. Identification of volatile components in basil ( L.) and thyme leaves ( L.) and their antioxidant properties. Food Chemistry - FOOD CHEM. 2005;91:131-137.
5. Dhiman N, Bhasin A. Marjoram (Origanum majorana): An essential oil with potential pharmacological properties and health benefits. The Pharma Innovation Journal. 2022;(11(7)):4454-4460.
6. Oregano Benefits, Nutrition, Side Effects and Recipes - Dr. Axe. Accessed January 24, 2024. https://draxe.com/nutrition/oregano-benefits/
7. Nagoor Meeran MF, Javed H, Al Taee H, Azimullah S, Ojha SK. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Frontiers in Pharmacology. 2017;8. Accessed January 22, 2024. https://www.frontiersin.org/articles/10.3389/fphar.2017.00380
8. Thymol | 89-83-8. ChemicalBook. Accessed January 24, 2024. https://www.chemicalbook.com/ChemicalProductProperty_EN_CB6672399.htm
9. Kowalczyk A, Przychodna M, Sopata S, Bodalska A, Fecka I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules. 2020;25(18):4125. doi:10.3390/molecules25184125
10. Bairwa R, Sodha RS, Rajawat BS. Trachyspermum ammi. Pharmacogn Rev. 2012;6(11):56-60. doi:10.4103/0973-7847.95871
11. Marchese A, Arciola CR, Barbieri R, et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials (Basel). 2017;10(8):947. doi:10.3390/ma10080947







I love this herbal information